Today (November 18, 1962) is Memorial Day of Nobel Prize-winning Niels Bohr for discovering the motions of electrons in the atom and their properties.


Niels Henrik David Bohr was born
on October 7, 1885, in Copenhagen, Denmark. His father was a worshiper of the
Christian War, the Ultimate sect of Christianity. He was a Professor of
Physiology at the University of Copenhagen. Mother Ellen came from an
influential Jewish family in Adler. Niels Bor's brother, Harald Bohr, was a
mathematician and Danish national footballer. Niels is a war footballer and
enthusiast. Both of them excelled in education under the care of their mother,
who came from an academic family, and their father, who excelled in the field
of physiology. Their upbringing contributed to their genius. Niels Bohr first
completed his matriculation in 1903 at the age of seven at the Camelham Latin
Grammar School. He later attended the University of Copenhagen.

Educated under the guidance of
the eminent professor of physics, Christian Christiansen, Niels completed his
master's degree in physics in 1909. He received his PhD in 1911. While a
university student in Copenhagen, he studied philosophy with his father's
friend, Harold Hopping, and with a degree in astronomy and mathematics. But in
1905 the Danish Academy of Sciences and Letters announced a scientific research
competition. It is a principled and theoretical solution to the area traction
that occurs in the oscillating fluid. To participate in this gold
medal competition, he did several recipe studies on the force of the liquid
surface in his father's laboratory. He also found a solution. His scientific
essay won the prize. This led him to drop out of philosophy and choose physics.
These research notes were published as a book in 1908 by the Royal Society.
His doctoral dissertation on the
properties of metals in terms of electron theory is ideologically the best
possible. These are in line with Planck's ideas about the radioactive particle
block. Later studies conducted under the guidance of the renowned J.J. Thomson
at the Cavendish Laboratory in Cambridge for his dissertation training helped
him in many ways. At the same time, he focused on ideological studies. Then in
1912, Ernest Rutherford studied at the University of Manchester in England. His
research was intensified in this study. Paved the way for dispelling the
fundamental doubts of radiation policy. In 1913, Ernest Rutherford first
proposed the concept of a war model (war model) in which the electrons inside
the atom revolve around the nucleus, based on their ideological principles.
Niels Bohr was the first to share that an electromagnet could emit light of certain energy from its high energy belt and jump into the lower energy belt.
This is one of the concepts underlying quantum theory.

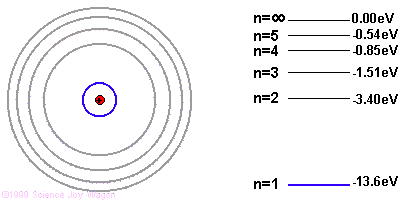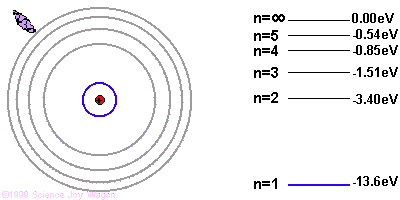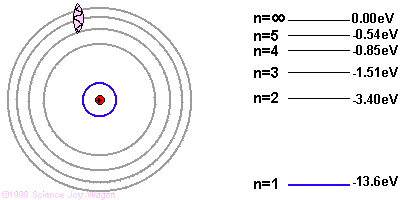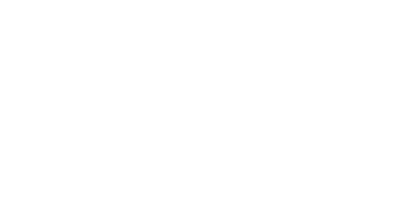
In 1913 his dissertation was published in the journal Philosophy. He carried out further research based on his ideas about the nucleus of the atom discovered by Rutherford. His research provided guidance on the subject of Planck's particle volume mechanics and provided some further explanations. His ideas still hold a special place in ideological physics today. His studies gave full shape to the structure of the atom. Heisenberg's ideas were later added in 1925, which shed light on the physical and chemical properties of the elements. The line spectrum is based on a few assumptions about quantum kinetics. They are as follows

1. An atom can only exist at a few
separate energy levels. An atom can release or inhale electromagnetic energy
according to the differences in these energy levels. These energy levels are
called "stationary states".
2. The vibration of the
electromagnetic wave caused by these energy changes is only a certain number!
This can be deduced from the following equation. E2-E1 = hf
h = is the Planck constant
f = frequency of the
electromagnetic wave
This is most difficult in those
days.

In 1913 he gave lectures on his
studies at the University of Copenhagen. He did similar work at Victoria
University in Manchester in 1914-16. In 1926 he was appointed Professor of
Ideology at the University of Copenhagen. From 1920 until he died in 1962 he
was president of the Institute for Theoretical Physics, which he founded at the
University. His thesis on the nuclear system earned him the world-famous Nobel
Prize in 1922. Since the 1930s his studies have focused on the nuclear
structure of the atom and the material transformations and decay that occur in them.
He provided a variety of explanations for the nuclear force exerted on the
smallest part of the nucleus by the atom and its effects.

The liquid droplet model system
gave a complete shape to the nucleus of this type of atom. This fluid droplet
principle helped to understand the nature of nuclear fission. Niels Bohr's
ideas were also instrumental in the ideological studies of Brish and Meitner
when they discovered the fission of uranium in 1939 by Hahn and Starman. Niels
Bohr's research has been published in approximately 115 books. Enmark came
under Nazi control during World War II. So Niels fled to war-torn Sweden. There
he spent two years studying the nuclear program. He also focused on the
peaceful use of nuclear physics and the political problems caused by nuclear
weapons. He acted with an open mind to find solutions to problems between
nations. His comments were published in 1950 in the book "Open Letter to
the United Nations".


His final focus turned to
molecular biology. His comments on this were published in the book "Light
and Life Revisited" after his death. He was a member of the Royal
Societies of many countries. Postage stamps have been issued in his honour.
Banknotes engraved with his image (in the value of the country) were also issued.
Renowned Danish scientist who gave basic ideas in the field of physics,
especially in nuclear science. Who discovered the motions of electrons in the
atom and its properties and gave full shape to the structure of the atom. He
was a mentor to many eminent physicists of the 20th century and was a
scientific associate in Copenhagen, Denmark, where he lived with many scholars.
His theory of quantum theory with Einstein is well known. He is known as one of
the greatest scientists of the 20th century. Nobel laureate Niels Enrique David
Bohr died in Copenhagen, Denmark on November 18, 1962, at the age of 77.
Source By: Wikipedia
Information: Dr. P. Ramesh, Assistant
Professor of Physics, Nehru Memorial College, Puthanampatti, Trichy.


.jpg)

No comments:
Post a Comment