Today (August 9, 1776) is the birthday of Amedeo Avogadro, who discovered the molecule of gases and Avogadro's law.

Lorenzo Romano Amedeo Carlo Avogadro was born on August 9, 1776, in Turin, Sardinia, Italy. He began his degree and training in ecclesiastical law in his late 20s. Soon, he devoted himself to physics and mathematics. In 1809 he began teaching at Lyso High School in Versailles. His family lived there and owned some property. In 1811, he published an article entitled Esai d'Un Manier de Determiner fewer mass cousins des Molecules elements des Corps, and les ratios "Molecules of bodies and the ratios they enter into these combinations". This is based on the hypothesis of Avocado. Avocado submitted this article to Jean-Claude Telamateri's Journal de Physic, de Simi et de Historic Nature ("Journal of Physics, Chemistry and Natural History").

In 1820, he became a professor of physics at the University of Turin. Avacado was active in the revolutionary movement in March 1821. As a result, in 1823 he allowed this interesting scientist to retire from rigorous teaching duties to better focus his research. Finally, King Charles Albert presented a constitution in 1848. Before this, Avocado was recalled to the University of Turin in 1833. There he taught for another twenty years. To pay homage to Avocado's contribution to molecular theory, the Avocado constant for the number of molecules in a mole of material is exactly 6.02214076 × 10^23 mol^- 1. The Avocado constant is used to calculate the results of chemical reactions. This allows chemists to determine the amount of material produced in a given reaction to a large degree of accuracy.


Avogadro's Number is a constant used in chemical calculations. This is a huge number. An avatar number is the total number of molecules or atoms or ions in a substance the size of a mole. In short, the avocado number is precisely the total number of atoms found in 12 g of carbon-12. The number of molecules in an object the size of a mole is always 6.02214X 10^23 mol^-1. The actual form of this number is abbreviated in mathematical form. 602 300 000 000 000 000 000 000. For example, one mole contains 18 grams of water, 180 grams of glucose, 44 grams of carbon dioxide, 32 grams of oxygen, and two grams of hydrogen molecules. That is the Avogadro number.


Johann Joseph Loshmit first calculated the value of the avocado constant. The number of particles in a mole is sometimes referred to as the loshmid number in German-speaking countries. Avocado developed this hypothesis in 1808 after Joseph Louis K.-Lusak published his law on blocks (combining gases). The biggest problem Avocado has to solve is the confusion regarding atoms and molecules. One of his most important contributions is to distinguish clearly from one another by stating that gases are made of molecules and that these molecules are made of atoms. (For example, John Dalton did not consider this possibility.) Avocado did not actually use the word "atom" because the terms "atom" and "molecule" were used almost indistinguishably. He believed that there were three types of "molecules", including the "basic molecule" ("atom"). Also, because of the difference in weight, he paid more attention to the mass definition.
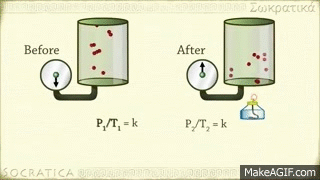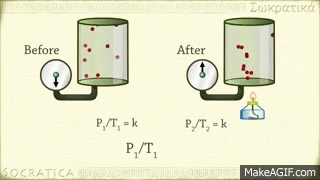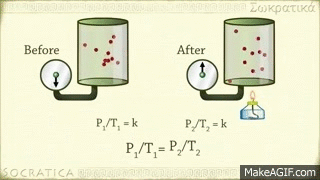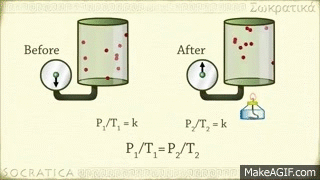
Avogadro's law is a gas law proposed by Avogadro. Avagadro said that different gases of the same capacity (equivalent) enclosed in the same shaped vessel would have the same number (atomic number) of molecules at the same temperature and at the same pressure. This discovery is known by his name as the fate of the Avocado. Amedeo Avogadro, best known for his discovery of the molecule of gases and Avogadro's law, died on July 9, 1856, at the age of 79 in Italy.
Source: Wikipedia
Information: Dr. P. Ramesh, Assistant Professor of Physics, Nehru Memorial College, Puthanampatti, Trichy.
Get information like this
Get information like this
https://t.me/joinchat/jpqj3jQLN51kYTk9
Join Telegram Group.
https://chat.whatsapp.com/HHC5m0Jz3Ue1E8ilgta0YT
Join WhatsApp Group
Thanks.
Also, Read
🛑👍 CSIR-NET Physics Materials and Problems
🛑📕 21 GB and Hundreds of Physics E-Books Collection.
🛑🛥️ How does an Electric Motor work? (DC Motor).
🛑🤹♂️ Science Academies' Summer Research Fellowship Programme for Students and Teachers 2022.
🛑🔌 How does a Transformer work - Working Principle electrical engineering.
🛑🎙️ Transistors Explained - How transistors work.
🛑🔥⚡ How Thermocouples Work - basic working principle.
🛑🔌 Voltage Explained - What is Voltage? Basic electricity potential difference
🛑🔌 What is CURRENT– electric current explained, electricity basics.
Also, Read
🛑👍 CSIR-NET Physics Materials and Problems
🛑📕 21 GB and Hundreds of Physics E-Books Collection.
🛑🛥️ How does an Electric Motor work? (DC Motor).
🛑🤹♂️ Science Academies' Summer Research Fellowship Programme for Students and Teachers 2022.
🛑🔌 How does a Transformer work - Working Principle electrical engineering.
🛑🎙️ Transistors Explained - How transistors work.
🛑🔥⚡ How Thermocouples Work - basic working principle.
🛑🔌 Voltage Explained - What is Voltage? Basic electricity potential difference
🛑🔌 What is CURRENT– electric current explained, electricity basics.
.jpeg)
.png)
.png)
No comments:
Post a Comment